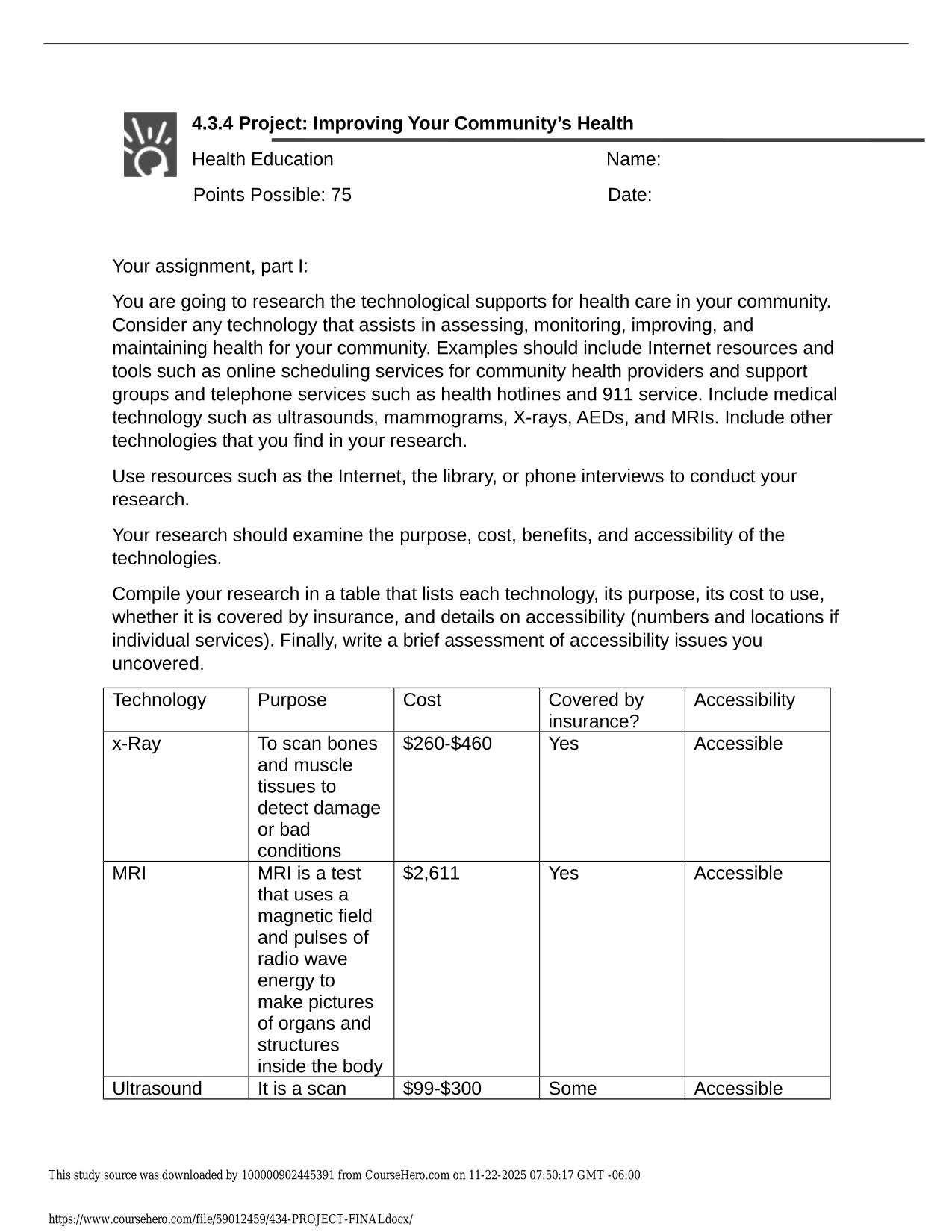
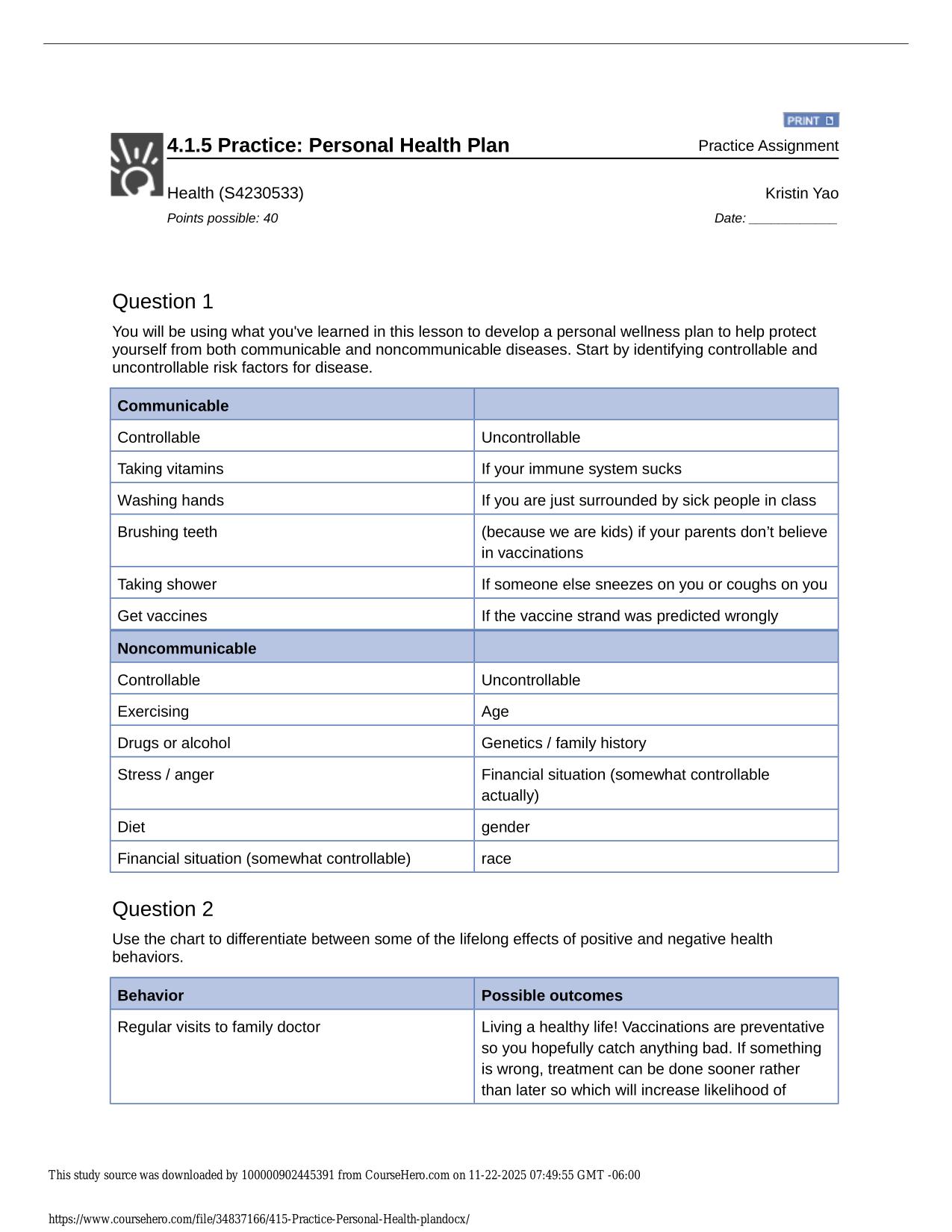
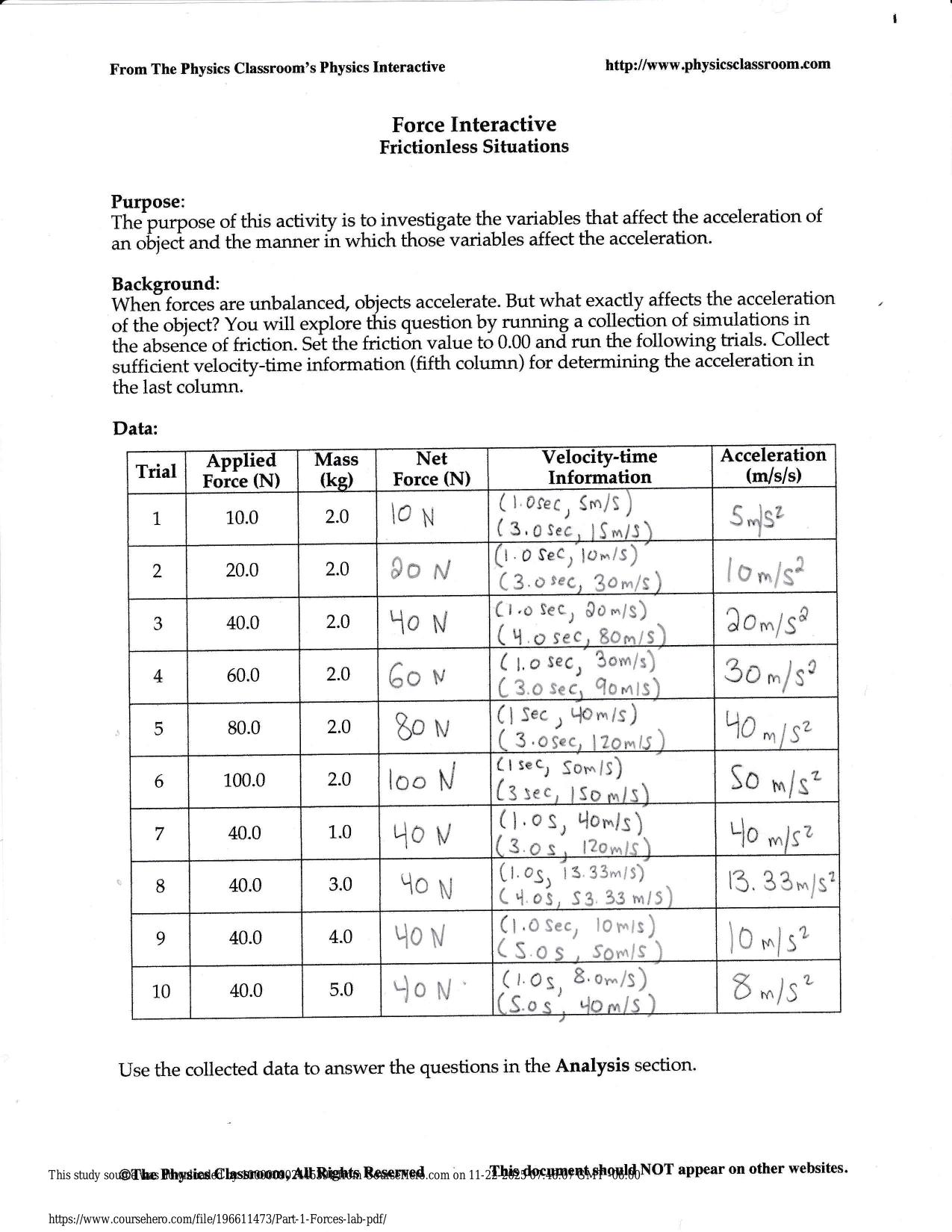
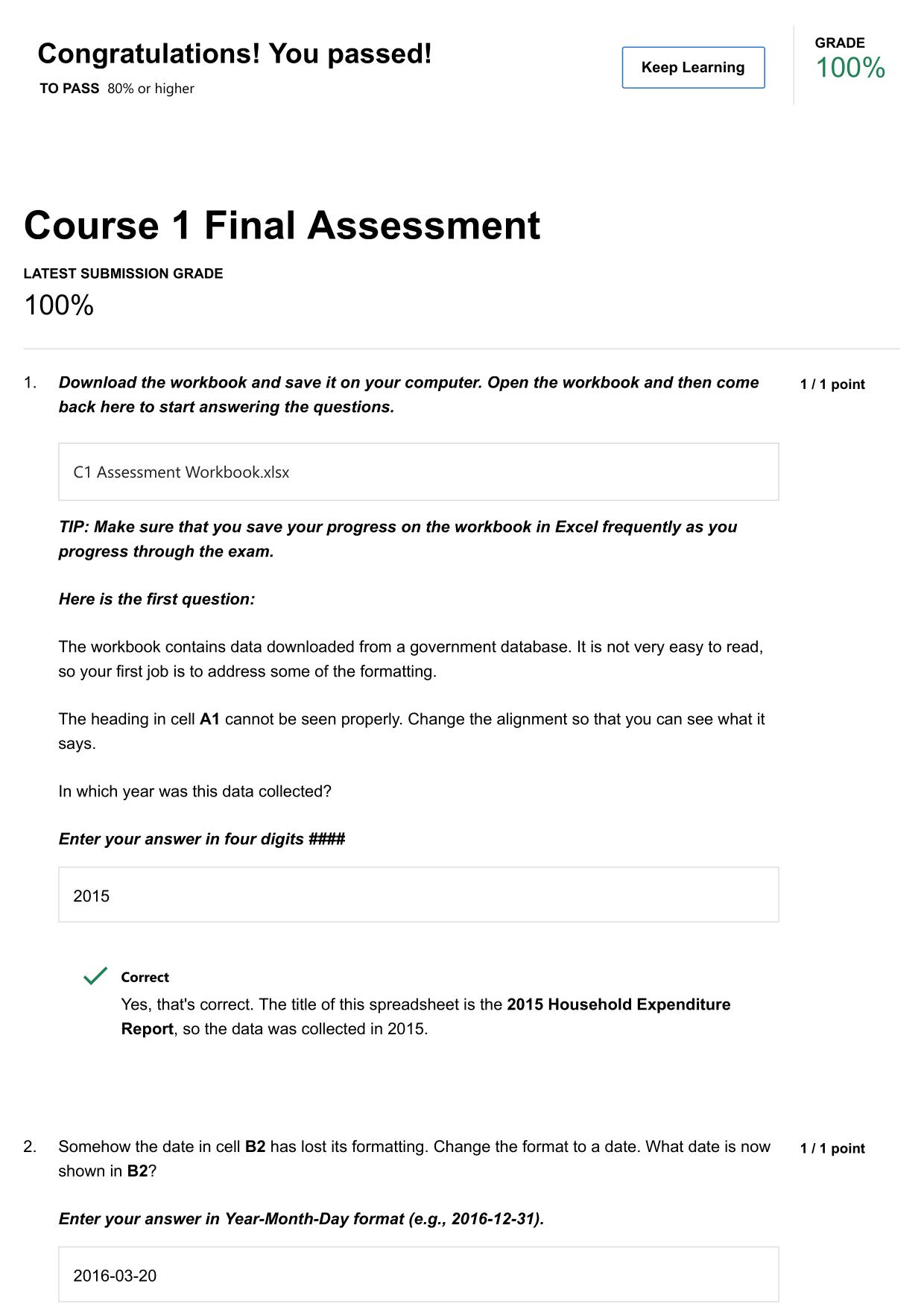
CITI GCP TRAINING ACTUAL 2025/2026 CORRECTLY ANSWERED
Course:
CITI
Institution:
CITI
CITI GCP TRAINING ACTUAL 2025/2026 QUESTIONS AND 100% CORRECT ANSWERS ICH E6 has broader requirements than FDA or HHS concerning confidentiality of medical records and access by third parties. If investigators are complying with ICH E6 guideline, the...
After purchase, you get:
✅ Instant PDF Download
✅ Verified answer explanations
✅ Refund if not Satisfied
✅ Prepared for 2025/2026 test cycle
Overview
Each section begins with foundational ideas and gradually introduces more challenging concepts for balanced progression. This building-block approach ensures you have the necessary basics before tackling advanced material. Learners appreciate how each concept naturally flows from what came before, creating a cohesive learning experience. The thoughtful sequencing prevents knowledge gaps that can undermine your self-assurance and performance.
Who Is This For?
Anyone preparing for CITI GCP TRAINING ACTUAL / CORRECTLY ANSWERED, including adult learners and working professionals, will benefit from the methodical layout of this resource. Many users report feeling better prepared after working through the materials. The logical progression builds understanding step by step.
Related Keywords
Detailed Study Description
Frequently Asked Questions
Document Information
| Uploaded on: | October 27, 2025 |
| Last updated: | November 17, 2025 |
| Number of pages: | 37 |
| Written in: | 2025/2026 |
| Type: | Exam (elaborations) |
| Contains: | Questions & Answers |
| Tags: | CITI GCP TRAINING ACTUAL 2025/2026 QUESTIONS AND 100% CORRECT ANSWERS ICH E6 has broader requirements than FDA or HHS concerning confidentiality of medical records and access by third parties. If investigators are complying with ICH E6 guideline, they must: - Answer -Clearly disclose to subjects in the informed consent form that the monitor, auditor, IRB/IEC, and the regulatory authorities may have access to the subject's medical records. ICH (2016) E6 Section 4.8.10(n) states that the informed consent should indicate that "the monitor(s), the auditor(s), the IRB/IEC, and the regulatory authority(ies) will be granted direct access to the subject's original medical records for verification of clinical trial procedures and/or data, without violating the confidentiality of the subject, to the extent permitted by the applicable laws and regulations and that, by signing a written informed consent form, the subj |
Seller Information

AdelineJean
User Reviews (0)
Exam (Elaborations)
$10.00
Bundle Deal! Get all 11 docs for just $24.00
Add to Cart
100% satisfaction guarantee
Refund Upon dissatisfaction
Immediately available after purchase
Available in Both online and PDF
$10.00
| 0 sold
Discover More resources
Available in a Bundle
Inside The Document
CITI GCP TRAINING ACTUAL 2025/2026 QUESTIONS AND 100% CORRECT ANSWERS ICH E6 has broader requirements than FDA or HHS concerning confidentiality of medical records and access by third parties. If investigators are complying with ICH E6 guideline, they must: - Answer -Clearly disclose to subjects in the informed consent form that the monitor, auditor, IRB/IEC, and the regulatory authorities may have access to the subject's medical records. ICH (2016) E6 Section 4.8.10(n) states that the informed consent should indicate that "the monitor(s), the auditor(s), the IRB/IEC, and the regulatory authority(ies) will be granted direct access to the subject's original medical records for verification of clinical trial procedures and/or data, without violating the confidentiality of the subject, to the extent permitted by the applicable laws and regulations and that, by signing a written informed consent form, the subject or the subject's legally acceptable representative is authorizing such access." The FDA regulations at 21 CFR 50.25(a)(5) (Protection of Human Subjects 2016) state only that in seeking informed consent, the following information shall be provided to each subject:. . . (5) A statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained and that notes the possibility that the Food and Drug Administration may inspect the records. While it is true that data sent out of the U.S. loses certain federal protections, this statement is not required. The possibility of hacking data is a risk that should be addressed in the study design and conduct. Non-disclosure forms are not required for communications with primary care providers. What is the status of ICH in U.S.? - Answer -It is a FDA guidance. After the ICH E6 guideline was finalized, several countries adopted it as law. In the United States, however, the FDA adopted the ICH E6 only as guidance. Therefore, the ICH E6 guideline does not have the force of law in the United States and is not a regulation. In the Federal Register notice, FDA stated that the ICH E6 guideline "does not create or confer any rights for or on any person and does not operate to bind FDA or the Need assistance on Online classes, Exams & Assignments? Reach out for instant help!! Full Course Assistance, Plagiarism-free Essay Writing, Research Paper, Dissertation, Discussion Posts, etc…. Confidential & Secure services. Tutors are available for all subjects! Email now at: tutorjean01@gmail.com public. An alternative approach may be used if such approach satisfies the requirements of the applicable statutes, regulations, or both" (HHS and FDA 1997, 25692). Therefore, compliance is voluntary, but as with any published FDA guidance, compliance is considered part of good clinical practice. Regarding subject receipt of a signed and dated copy of the consent forms, which is true about FDA regulations? - Answer -The FDA regulations allow subjects or the legally acceptable representatives (LARs) to receive either a signed or unsigned copy. The FDA regulations allow subjects to receive either a signed or unsigned copy. ICH E6 Section 4.8.11 requires that the subject or the legally acceptable representative (LAR) receive a copy of the signed and dated written informed consent form. The FDA (1998) regulations allow subjects to receive either a signed or unsigned copy. To be in compliance with ICH E6 guideline, the investigator should include a statement in the consent form that the subject will receive a signed and dated copy of the consent form. Persons obtaining consent must then ensure that this procedure is followed. The new ICH E6(R2) integrated addendum requires sponsors to implement systems to manage quality throughout all stages of the trial process. The system should use a risk-based approach including which of the following? - Answer -Identification of study risks to determine which may safely be omitted from continual monitoring. ICH (2016) E6 Section 5.0.4 states that the sponsor should decide which risks to reduce and/or which risks to accept. The approach used to reduce risk to an acceptable level should be proportionate to the significance of the risk. Risk reduction activities may be incorporated in protocol design and implementation, monitoring plans, and agreements. Routine scheduled audits of study documentation whether on-site or remote are not considered fully responsive to the need for continuous monitoring of data under a proactive risk-based approach. While data from Case Report Forms may be selected for ongoing monitoring, there is no ICH template and a “one-size-fits-all” approach Need assistance on Online classes, Exams & Assignments? Reach out for instant help!! Full Course Assistance, Plagiarism-free Essay Writing, Research Paper, Dissertation, Discussion Posts, etc…. Confidential & Secure services. Tutors are available for all subjects! Email now at: tutorjean01@gmail.com
CourseHero & Studypool Unlocks
Get Unlocked CourseHero and Studypool documents files instantly to your email, simply by pasting your link and clicking "Unlock Now". Learn more on how to unlock here.